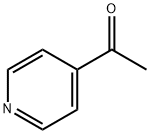
4-Acetylpyridine
Product Name: 4-Acetylpyridine
Synonyms: METHYL 4-PYRIDYL KETONE;4-ACETYLPYRIDINE;AKOS BBS-00003233;gamma-Acetylpyridine;1-(4-pyridinyl)-ethanon;1-(4-Pyridinyl)ethanone;1-pyridin-4-yl-ethanone;4-acetyl-pyridin
CAS: 1122-54-9
MF: C7H7NO
MW: 121.14
EINECS: 214-350-9
4-Acetylpyridine Chemical Properties
Melting point 13-16 °C(lit.)
Boiling point 212 °C(lit.)
density 1.095 g/mL at 25 °C(lit.)
refractive index n20/D 1.529(lit.)
Fp >230 °F
storage temp. Inert atmosphere,Room Temperature
solubility Chloroform, Ethyl Acetate
pka pK1: 3.505(+1) (25°C)
form Liquid
Specific Gravity 1.110 (20/4℃)
color brown-yellowish
Water Solubility insoluble
BRN 107629
InChIKey WMQUKDQWMMOHSA-UHFFFAOYSA-N
LogP 0.480
CAS DataBase Reference 1122-54-9(CAS DataBase Reference)
NIST Chemistry Reference Ethanone, 1-(4-pyridinyl)-(1122-54-9)
EPA Substance Registry System 4-Acetylpyridine (1122-54-9)
Safety Information
Hazard Codes Xi,Xn
Risk Statements 36/37/38-36/38-22
Safety Statements 26-36-37/39
WGK Germany 3
RTECS OB5426000
F 8
Hazard Note Irritant
TSCA Yes
HS Code 29333999
4-Acetylpyridine Usage And Synthesis
Chemical Properties clear light yellow to brownish liquid
Uses 4-Acetylpyridine is a acetylated pyridine used in the preparation of nitrogen containing bicyclic heterocycles and other organic compounds. 4-Acetylpyridine is also present in mainstream cigarette smo ke.
Uses 4-acetylpyridine on reaction with – and 4-formylpyridine (H3FoPyS, H4FoPyS) yields semicarbazones. Reaction of acetylpyridine with thiophene-2-carboxaldehyde afforded the 2-chloro-6-ethoxy-4-β-(2-thienyl)acryloylpyridine, which was reacted with malononitrile in refluxing ethanol in the presence of piperidine as a catalyst to afford the cyanoaminopyrane derivative.
General Description Dark amber liquid.
Air & Water Reactions Soluble in water.
Reactivity Profile 4-Acetylpyridine is incompatible with strong oxidizing agents and strong reducing agents. . Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Fire Hazard 4-Acetylpyridine is probably combustible.
Related products
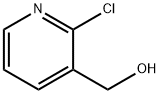
4-Chloronicotinic acid CAS:10177-29-4 C6H4ClNO2
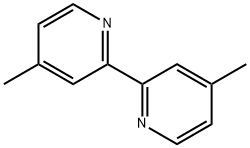
4,4′-Dimethyl-2,2′-bipyridyl 1134-35-6
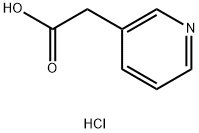
3-Pyridylacetic acid hydrochloride 6419-36-9
5-Bromo-2-pyridinecarboxylic Acid 30766-11-1
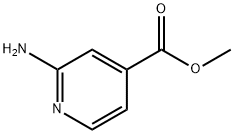
Methyl 2-aminopyridine-4-carboxylate
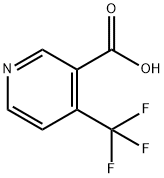
4-(Trifluoromethyl)nicotinic acid
Methyl syringate
| Product Name: | Methyl syringate |
| Synonyms: | METHYL 3,5-DIMETHOXY-4-HYDROXYBENZOATE;METHYL-4-HYDROXY-3,5-DIMETHOXYBENZOATE;METHYL SYRINGATE;3,5-DIMETHOXY-4-HYDROXYBENZOIC ACID METHYL ESTER;methyl syringoate;Syringic acid monomethyl ester;4-Hydroxy-3,5-Dimethoxybenzoic Acid Methyl Ester;Benzoic acid, 4-hydroxy-3,5-dimethoxy-, methyl ester |
| CAS: | 884-35-5 |
| MF: | C10H12O5 |
| MW: | 212.2 |
| EINECS: | 429-050-5 |
| Product Categories: | Pharmaceutical Intermediate;Aromatic Esters;Acids & Esters;Anisoles, Alkyloxy Compounds & Phenylacetates;Phenols |